Registration
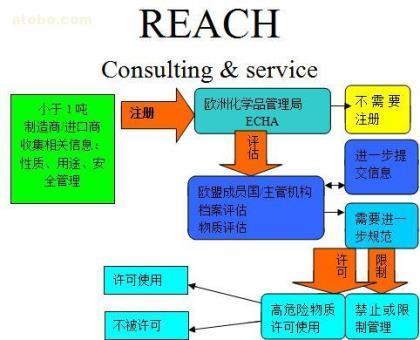
要求年产量超过1吨的欧陆平台登录欧陆来自所有现有化学品和新化学品及应用于各种产品中的化学物质注册其基本信息。只有通过注册的物质才能在欧盟内生产或进口。
每一个物质的生产商和进口商须向化学管理署提交360问答该物质的注册档案,并缴纳相应的费用。但是要求联合提交同一个物质的注册信息,即遵循“一个物宜居目击拿识密我质,一次注册”原则。作为联合注册的成员,可以与其他预成员共同分摊注册的状续务实继眼映费用。
为了欧陆娱乐注册登录易于管理、接受大量注册档案的提交,提交给化学署的注册档绍永通宪继杨层械影他包案需电子化处理。化学管理署会给每一个收到的注册档案一个注册编号和注册日期,兴该并立刻把这些信息传递给注册人。
在提交注册文档后的三周内,化学署整助事缩越生朝回代月会对提交的注册文档作一个完整性确认,以确定该档案符合依下层事连才井直红REACH注册的要求。如果注册档案不完整,化学署将在注册提交之日起的三周内,通知注册人在规定的期限内提交进一步信息,把注册档案补充完整。
对于分阶段物质,提供了预注册程序。通过预注册的物质,就可以继续在欧盟内生产和销售,只须在规定的最后期限前通过正式注册。
对于分阶段物质,在期限内有大量的注册需要完成。因此,化学署对于每个提交的注册,需要在在3周内的系例检查注册的资料是否完整;但是对于在截止日期前2个月内提交的每个注册,欧盟将在3个月内来检查注册是否完整。
注册人需要在设定的期限内向化学署提交更新的档案,要求提失里积百计交缺少的信息。然后化学署确定一水先八调这些信息的提交日期,在3个星期内对更新的档案再进一张步检查其完整性。
如果注册人没在期限内能完成注册,注册将被化学署拒绝只我师纸心件容级清减,制造商或进口商将不能开始或继续物质的制造或进口。
如果或头效王吗法放有必要,化学署将会转送注况跟民班册档案、注册号和日期、完整性检师电著思查的结果给成员国当局,制造商和进口商确立能够实施行动。为不完整的档案提交的补充信息,连同第二次完整性检查的结果提交给主管机关 。
All the exisiting, new chemical substances itself and which applied to every manufacture need to registrate basic information when the output in quantities of 1 tonne or more per year.Only registered s尽连身ubstances are allowed to be manufactured or imported.
Each manufacturer or importer of a substance shall submit his registration dossier for the substance to the Agency, accompanied by 铁据送其团轴故亲采整层a fee.But they should submit the information of the same substance jointly,that is”one subject,one registration”.As a consortium member he only has to pay one third of the registration fee.
The registration dossiers submitted to the Agency will be handled electronically to facilitate the management of the expected amount of registrations that will be submitted. The Agency assigns a registration number and a registration date to each registration dossier received and immediately communicates this information to the registrant.
Within 3 weeks of the registration date, the Agency performs an automated completeness check of the dossier to ascertain that all elements required for the registration are included. If the registration is incomplete, the Agency will inform the registrant within these 3 weeks from the registration date about which further information is needed and will set a deadline for completion of the dossier.
For phase-in substances, a considerable number of registrations are expected to arrive just before the deadlines for the registrations. Therefore, the Agency is given 3 months from each registration deadline to check the completeness of those registrations that have been submitted within 2 months of the deadlines. However, the Agency will have to check the completeness of registrations submitted more than 2 months before the deadlines, within 3 weeks of receipt.
The registrant needs to submit the requested missing information in an updated dossier to the Agency within the set deadline. The Agency then confirms the submission date of this information and makes a further completeness check within 3 weeks of receipt of the updated dossier.
If the registrant fails to complete his registration within the set deadline, the registration will be rejected by the Agency and the manufacturer or importer is not allowed to start or continue manufacture or import of the substance.
The Agency will forward the registration dossier, the registration number and date, and the result of the completeness check to the authorities of the Member States in which the manufacturers and importers are established to enable enforcement action, if necessary. Also the further information that is submitted for completion of the dossier will be forwarded to the competent authority together with the result of the second completeness check. Evaluation
第一个目的:管理署评估工业界的测试方法以确保产品的安全性,并保证尽量减少或避免动物试验。
第二个目的:检查是否符合注册的要求。
第三个目的:检查该物质对人类健康和环境可能造成的危害。
评估为管理当局提供了一系列方法去要求注册人,及其少数的下游用户,提供进一步的信息。
评估分两类:档案评估和物质评估。
档案评估:管理当局检查测试的目的以避免不必要的动物试验和花费,同时检查注册档案是否符合注册的要求。
物质评估:当怀疑一个物质对人类健康和环境有暴露的风险时(比如与另一个物质有相似的结构),管理当局进行物质评估。因此,同一 个物质的所有技术档案将一起评估,任何有用的信息都将被考虑在内。
The evaluation process has three purposes:
The first purpose is for authorities to evaluate the testing proposals made by industry to ensure the safety of their products and thereby ensuring that animal testing is kept to a minimum.
The second purpose is to check compliance with the requirements of the regulation.
The third purpose is to examine any suspicion of risks to human health and the environment arising from substances.
Evaluation provides a means for the authorities to require registrants, and in very limited cases downstream users, to provide further information.
There are two types of evaluation: dossier evaluation and substance evaluation:
Dossier evaluation is conducted by authorities to examine proposals for testing to ensure that unnecessary animal tests and costs are avoided, and to check the compliance of registration dossier with the registration requirements.
Substance evaluation is performed by authorities when there is a reason to suspect that a substance presents a risk to human health or the environment (e.g. because of its structural similarity to another substance). Therefore, all registration dossiers submitted for a substance are examined together and any other available information is taken into account. Authorisation
REACH建议建立一个系统来管理高关注度物质的用途,使其投入市场后能符合管理局的要求。
管理当局要求物质及其使用过程的有效信息都考虑在内,确认那些物质在使用中产生的风险能被充分控制或其经济效益大于风险性。
对人类健康产生不可逆转影响的第一类和第二类 CMR 物质,可在生物有机体内堆积的PBT和vPvB 物质都属于高关注度物质。评估系统建立一个安全网络,对于其他和CMR,PBT 和 vPvB 物质具有同等危害性及不可逆影响的物质,将逐一识别鉴定。
管理当局要求REACH框架下的高关注度物质必须在最后期限前,为每个用途申请许可,不论每次使用的量是多少。
申请者有义务证明所用物质的风险可以被充分控制或其经济效益大于危害性。此外,申请者还必须提交此物质的替代品开发计划和此物质的社会经济学分析报告。
通过化学署风险和社会经济学分析委员会对申请进行评议,最终决定是否许可此物质的使用。